Nigeria Makes Medical History as NAFDAC Approves Robotic Surgery
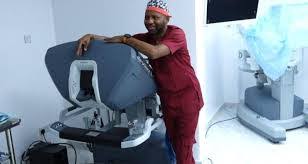
Nigeria has taken a major step in advanced healthcare after the National Agency for Food and Drug Administration and Control (NAFDAC) approved the Toumai robotic surgical system for clinical use.
The decision makes Nigeria the first country in West Africa to officially approve a robotic surgery platform, opening access to modern surgical care for millions across the sub-region.
The approval followed successful robotic surgeries carried out on November 22, 2025, at NISA Premier Hospital in Abuja. The procedures were performed by Dr. Obi Ekwenna of RoboMed, with both patients recovering quickly and leaving the hospital within two days, far sooner than with traditional open surgery.
NAFDAC’s clearance confirmed that the system is safe and effective for use in Nigeria. Dr. Ekwenna said the approval showed the agency’s strict review process and assured patients that the technology meets global standards, reducing the need for Nigerians to travel abroad for complex surgeries.
The Toumai system, produced by Shanghai-based MicroPort MedBot, allows doctors to perform delicate operations using high-definition 3D visuals and robotic arms.
The technology supports minimally invasive surgery, leading to smaller cuts, less pain, fewer complications and faster recovery for patients.
Following the approval, RoboMed announced plans to partner with more hospitals across Nigeria and beyond, while also setting up a training academy for local surgeons.
A public launch of the robotic surgical system is expected to take place in Abuja in January 2026.







